همکاران
مکملهای غذایی
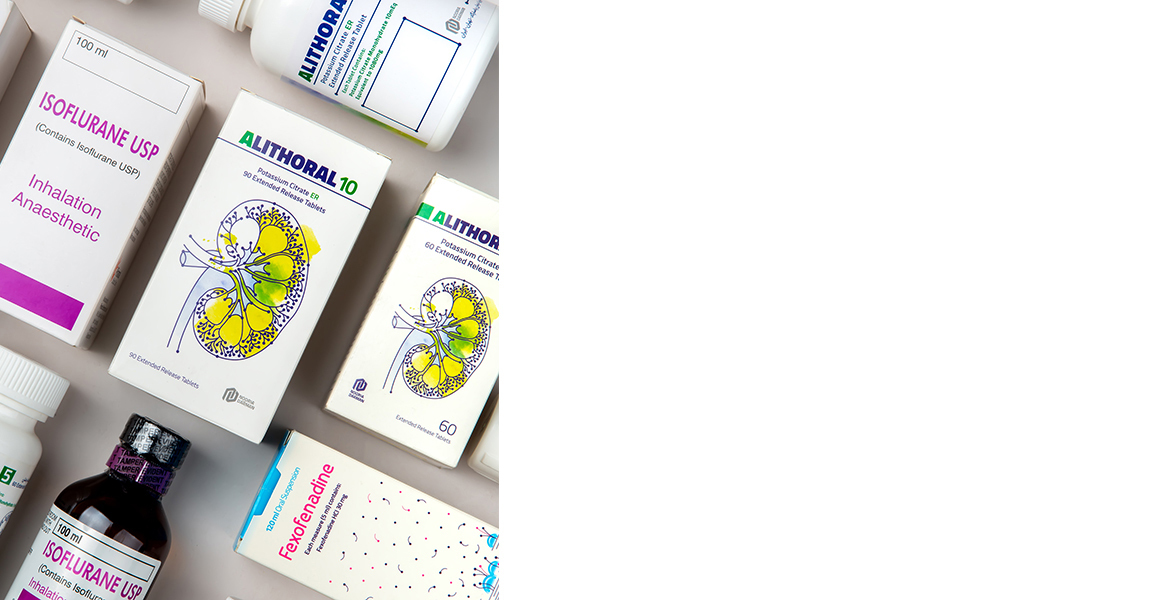
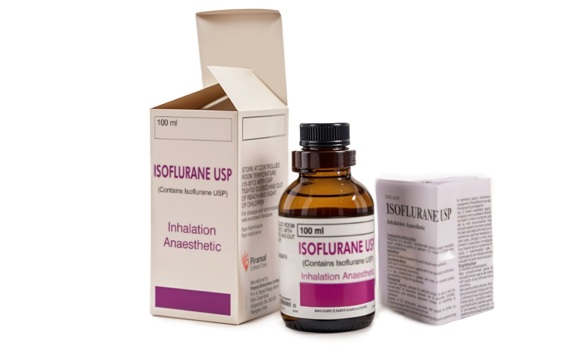
محصولات دارویی
مواد اولیه
®Pharmatose

بدون تست حیوانی

مواد اولیه با کیفیت

حلال
نشانهای محصولات
درباره ما
شرکت دارویی نوریا درمان پاسارگاد با بیش از 7 سال پیشینه فعالیت در حوزه صنعت مکمل و دارویی کشور:
- تیمی جوان و هدفمند
- توانمند در واردات، ثبت، تولید، بازاریابی و فروش محصولات دارویی و مکمل غذایی
- فعال در حوزه دارو, مکمل غذایی و مواد اولیه دارویی
- تاکید بر کیفیت محصولات
- تلاش مداوم بر گسترش فعالیت و سبد محصولات